FDA proposes plan to roll out annual COVID booster shots
Dr. Houman Hemmati discusses the data behind the FDA's proposal to make the COVID-19 vaccine schedule similar to that of flu shots on 'Fox News @ Night.'
A US Centers for Disease Control and Prevention (CDC) advisory group said Friday it isn’t recommending more than one annual coronavirus vaccine booster.
The working committee, which is part of the CDC’s Advisory Committee For Immunization Practices, found insufficient evidence that more than one shot a year would benefit older or immunocompromised people.
Annual shots are recommended by the CDC as the vaccine’s effectiveness diminishes over time, especially in more vulnerable populations - the shot generally wanes faster in older people compared ot younger adults - and the group said it recommends an annual booster campaign that would likely start later this year.
The Food and Drug Administration (FDA) outlined a plan for a yearly shot last month but an FDA committee said it wasn’t sure if yearly vaccines were the right move because there are still so may unknowns about the virus.
FDA TO PROPOSE YEARLY COVID VACCINES LIKE ANNUAL FLU SHOTS FOR AMERICANS
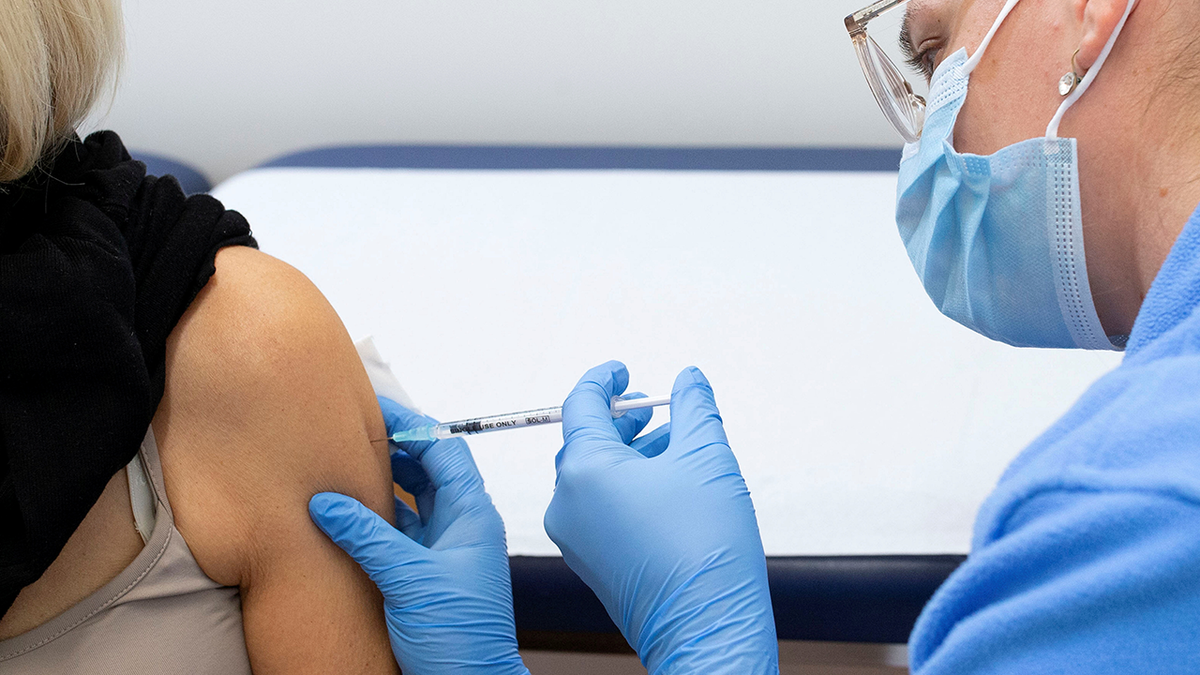
The CDC committee said there's insufficient evidence to recommend more than one annual COVID-19 shot. (Reuters/Arnd Wiegmann)
The Administration’s Vaccine and Related Biology Products Advisory Committee stressed that COVID-19 is not exactly like the flu, which is more seasonal regarding surges in infections.
YEARLY COVID VACCINE AS PROPOSED BY FDA? ‘CART BEFORE THE HORSE,’ SAYS DOCTOR
"We may or may not need annual vaccination," Dr. Cody Meissner, a Tufts University School of Medicine pediatrician, said, according to NBC News. "It’s just awfully early, it seems to me, in this process to answer that."
Earlier this month, the CDC added the COVID-19 vaccine to the child and adolescent immunization schedule.

The CDC committee said it recommends an annual COVID-19 booster shot campaign. (Reuters/Tami Chappell/File Photo)
The schedule, which is posted on the CDC's website, recommends that children between six months and 18 years old should receive two doses of the primary series between four and eight weeks apart — followed by a booster dose at least eight weeks later.
The schedule is a recommendation, not a requirement.
CLICK HERE TO GET THE FOX NEWS APP
Children who are "moderately or severely immunocompromised" should include a third dose in the primary series, says the CDC.
Reuters and Fox News' Melissa Rudy contributed to this report.

