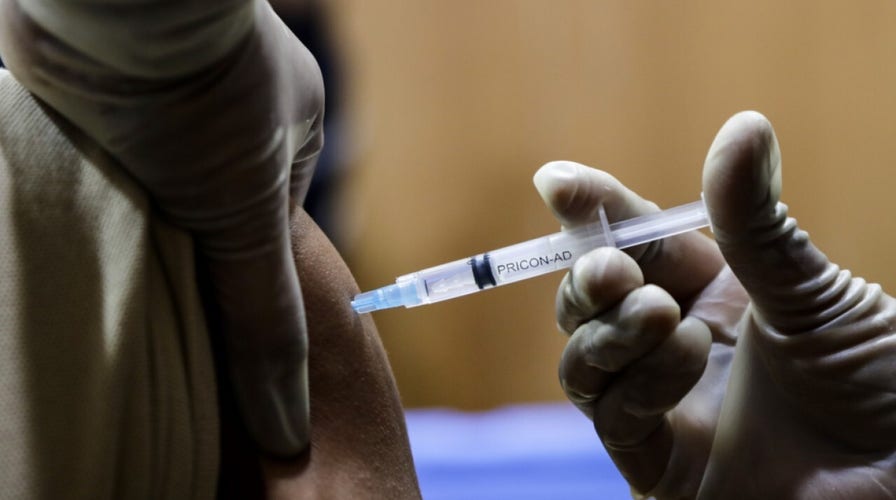
COVID booster may be available for immunocompromised adults
Fox News correspondent Peter Doocy has the latest on combatting the coronavirus pandemic on 'Special Report'
A Centers for Disease Control and Prevention (CDC) advisory committee is recommending moderate-to-severe immunocompromised individuals receive an mRNA COVID-19 booster dose. The Advisory Committee on Immunization Practices held the vote Friday, a day after the FDA expanded emergency use of Pfizer-BioNTech and Moderna’s COVID-19 vaccines to authorize a booster shot for certain immunocompromised patients.
The vote was 11 to 0.
The recommendation will now go before CDC Director Dr. Rochelle Walensky for final approval.
FDA GREENLIGHTS COVID-19 BOOSTER VACCINE FOR SOME IMMUNOCOMPROMISED PATIENTS
Committee members were tasked with discussing the following question:
Should vaccination with an additional dose of Pfizer-BioNTech COVID-19 vaccine (≥12 years) or Moderna COVID-19 vaccine (≥18 years) be recommended following a primary series in immunocompromised people, under an Emergency Use Authorization?
The panel met on Friday to discuss clinical recommendations regarding an additional dose for immunocompromised individuals. Growing evidence had suggested select immunocompromised patients mount a diminished protective immune response, even after two doses of vaccine. HIV and cancer patients, organ transplant recipients and those taking immunosuppressant drugs comprise about 2.7% of the U.S. adult population.
The panel said the additional dose should match the vaccine given during the initial series, but if it’s not feasible, another mRNA dose is permitted. The panel also recommended the additional dose be administered at least 28 days after completing the primary series.
Patients eligible for booster shots under the recommendation and amended emergency approval include those with moderate to severe immune compromise, such as solid organ transplant recipients, patients with advanced or untreated HIV infection and those taking high-dose corticosteroids and severely immunosuppressive cancer treatments, among others.
The FDA had not expanded authorization of the one-shot Johnson & Johnson vaccine on Thursday as an additional dose among immunocompromised patients due to insufficient data. However, the FDA and CDC are working to issue guidance on the matter.
Several studies were presented ahead of the vote, including research published in the New England Journal of Medicine on Wednesday among 120 organ transplant recipients who received a third dose of Moderna's vaccine, which indicated a substantial boost in neutralizing antibodies and T-cell counts, compared to a group receiving saline placebo.