First COVID-19 vaccinations begin in US
Reaction from Dr. Marty Makary on ‘America’s Newsroom.’
The long-awaited moment has finally arrived: The first doses of the highly anticipated coronavirus vaccine are officially here, with the very first jab administered on Monday to a critical care nurse in New York.
The rollout of Pfizer and BioNTech’s COVID-19 vaccine comes just days after the U.S. Food and Drug Administration (FDA) OK’d the companies’ request for emergency use, making it the first vaccine against the virus to receive such approval.
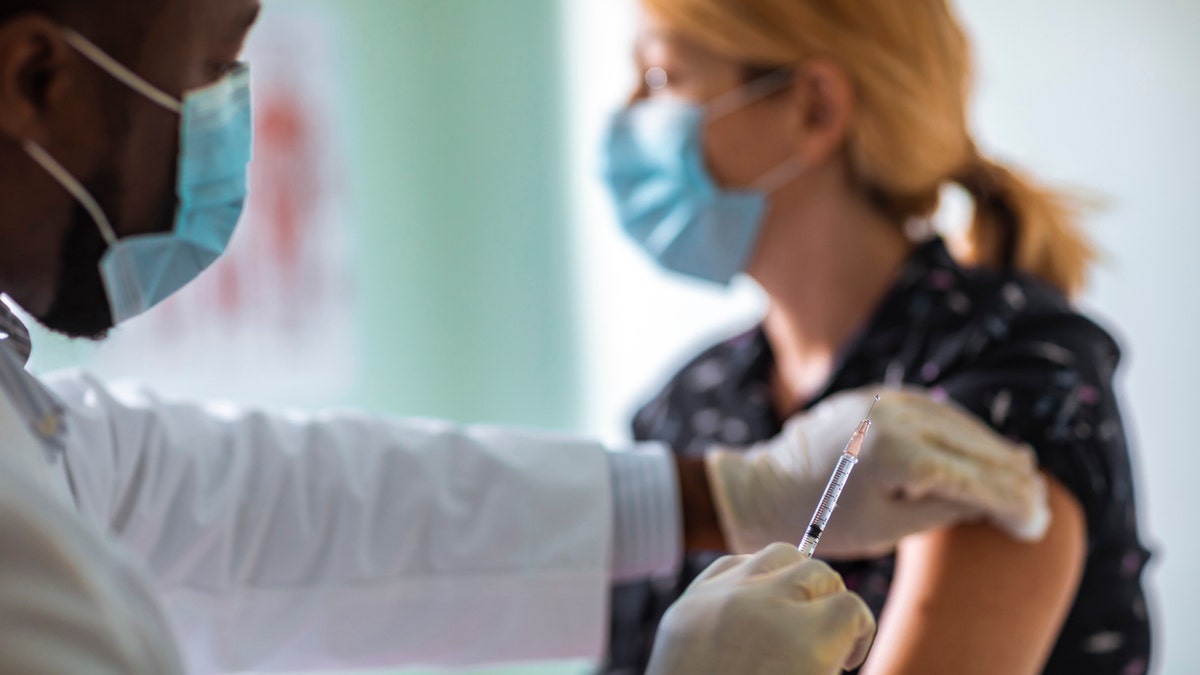
“We do know that people who have had COVID will have immunity for a period of months to years. We just don't know how long,” said Dr. John Whyte, the chief medical officer of the health care website WebMD, in an email to Fox News. (iStock)
The FDA is set to meet Thursday to review the Moderna vaccine. A third candidate, from Johnson & Johnson, which would require just one dose, is working its way through the pipeline. Behind that is a candidate from AstraZeneca and Oxford University. U.S. health experts are hoping a combination of vaccines will ultimately enable the U.S. to conquer the outbreak.
“This is the beginning of the end,” said U.S. Surgeon General Dr. Jerome Adams of the vaccine rollout during an appearance on “Fox and Friends” on Monday.
WHAT'S THE DIFFERENCE BETWEEN MRNA VACCINES AND CONVENTIONAL ONES?
The Centers for Disease Control and Prevention (CDC) has recommended that the vaccine first go to health care workers and residents and staff of long-term care facilities, with other groups — such as those 65 years of age and older and those with certain preexisting health conditions — likely to be included in the coming rounds.
But a muddled question remains: should those who have already contracted the novel coronavirus and recovered from it receive the vaccine? In short: It’s not totally clear — yet. But from what we do know, many experts are suggesting yes.
“We do know that people who have had COVID will have immunity for a period of months to years. We just don't know how long,” said Dr. John Whyte, the chief medical officer of the health care website WebMD, in an email to Fox News.
NY GIVES FIRST CORONAVIRUS VACCINE TO HEALTH CARE WORKER
He noted that if the novel virus, SARS-CoV-2, is like other coronaviruses, “it may be a couple of years,” he said of immunity.
Indeed: a study published in November — said to be the most comprehensive to date — suggested that immunity against the virus could last for at least six months, or perhaps a matter of years.
“We also think that immunity from the vaccine may be stronger than getting COVID and recovering,” added Whyte.
“Given limited resources, I expect that people who have had COVID will be ‘last in line’ since they have some protection compared to people who have had none,” he continued.
FIRST CORONAVIRUS VACCINE DELIVERIES OUT TO ALL 50 STATES IN US
In a Dec. 13 post — made just one day before the widespread rollout of the Pfizer vaccine — the CDC said that people who have already contracted and recovered from the deadly virus “may be advised to get a COVID-19 vaccine even if they have been sick with COVID-19 before.”
The federal health agency cited the “severe health risks” associated with the disease as a reason, as well as the possibility of re-infection.
“At this time, experts do not know how long someone is protected from getting sick again after recovering from COVID-19. The immunity someone gains from having an infection, called natural immunity, varies from person to person. Some early evidence suggests natural immunity may not last very long,” the CDC continued.
"We won’t know how long immunity produced by vaccination lasts until we have a vaccine and more data on how well it works,” it added. “Both natural immunity and vaccine-induced immunity are important aspects of COVID-19 that experts are trying to learn more about, and CDC will keep the public informed as new evidence becomes available.”
FDA APPROVES PFIZER'S CORONAVIRUS VACCINE FOR DISTRIBUTION
Dr. Nancy Messonnier, the director of the National Center for Immunization and Respiratory Diseases at the CDC, spoke to this issue last week during a fireside chat with the Aspen Institue.
When responding to a question if there could be any adverse side effects from taking the COVID vaccine after having contracted the virus, Messonnier said there was both a “theoretical response” and “practical response.”
“The scientists who know about this disease have worried about this — they don’t believe there is a serious harm [in taking the vaccine if you have already had and recovered from the virus],” she said.
“Practically, in the clinical trial, I understand that they excluded people who were infected at the moment that they enrolled in the trial, that there were some people in the clinical trial who had COVID in the past and they had antibody levels — and that is certainly something that the scientist who are going to be reviewing this data are going to be looking for,” she continued.
An estimated 10% of volunteers in the Pfizer and Moderna trials were thought to have been previously infected, though both companies didn’t actively recruit those who were symptomatic or had a known infection, Dr. Moncef Slaoui, the chief science adviser for Operation Warp Speed, previously said.
“Equally important as the question is, ‘What does this mean down the road? How do I know how long the vaccine is going to last and is it going to protect me forever?’ The truth is, we don’t know that — and this is not surprising. There are always things that we don’t know at the moment a vaccine is authorized and licensed. A clinical trial, as big as it is, is different [from] rolling out the vaccine to the entire public. That’s why it’s so important to study the vaccine — so we’re going to be carefully studying this vaccine to see how well it works in the public once implemented and also to be looking at those important questions,” added Messonnier during the fireside chat.
Dr. Anthony Fauci, the nation’s top infectious disease expert, had a more direct answer when speaking to this topic during a conversation with the Milken Institue last week.
CLICK HERE FOR COMPLETE CORONAVIRUS COVERAGE
“The answer is yes,” Fauci said of COVID-19 survivors receiving the vaccine. “Once you get infected with the virus, it isn’t certain how long that protection will be or whether or not you amounted good protection. So it isn’t a contraindication against the vaccine if you’ve already been infected.”
As of Monday morning, all 50 states plus the District of Columbia have reported confirmed cases of COVID-19, tallying more than 16,256,812 illnesses and at least 299,181 deaths.
The Associated Press contributed to this report.








































