FDA expected to approve Pfizer vaccine for teenagers
'Fox & Friends First' parent panel weighs in on adolescent vaccinations.
The U.S. Food and Drug Administration (FDA) is working to review Pfizer’s request to expand authorization for use of its COVID-19 vaccine among adolescents "as quickly and transparently as possible," an agency spokesperson confirmed to Fox News.
"The FDA’s review of Pfizer’s request to amend its emergency use authorization (EUA) in order to expand the age range for its COVID-19 vaccine to include individuals 12-15 years of age is ongoing," said Alison Hunt, FDA spokesperson, in an emailed statement. "We can assure the public that we are working to review this request as quickly and transparently as possible."
As of now, Pfizer’s vaccine is authorized for all adults aged 16 and over in its EUA.
FDA EXPECTED TO OK PFIZER VACCINE FOR TEENS WITHIN WEEK
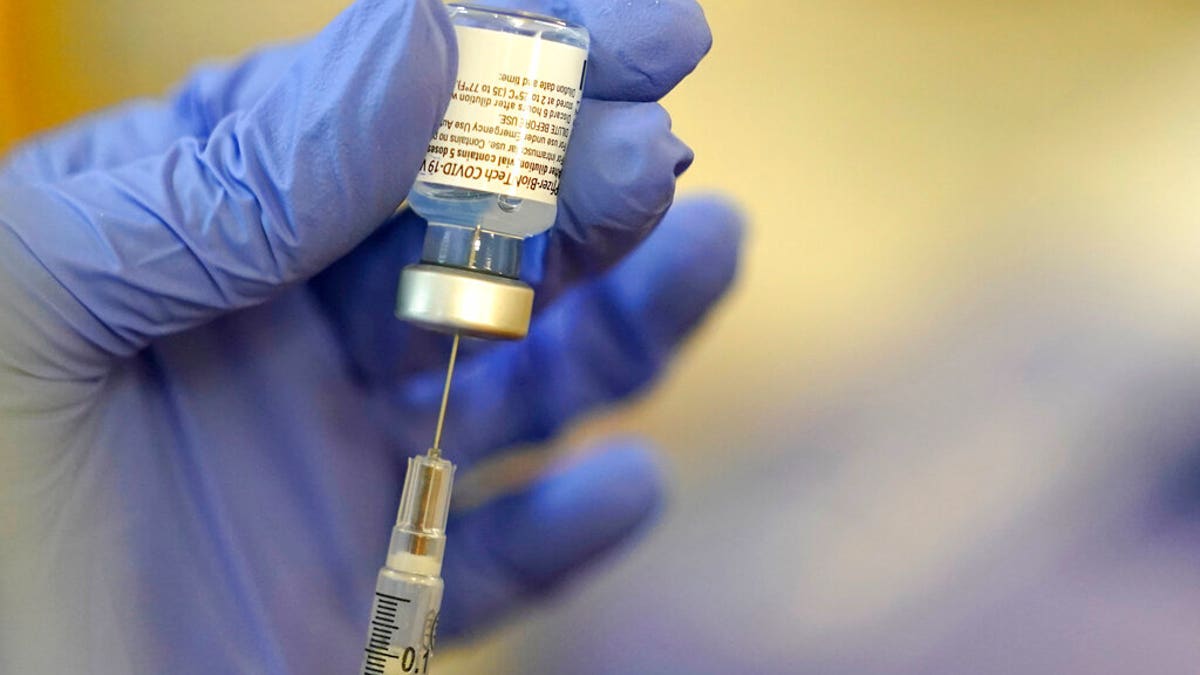
A pharmacist prepares a syringe of the Pfizer-BioNTech COVID-19 vaccine Friday, Jan. 8, 2021, at Queen Anne Healthcare, a skilled nursing and rehabilitation facility in Seattle. (AP Photo/Ted S. Warren)
The news comes as Canada on Wednesday authorized the Pfizer-BioNTech COVID-19 vaccine for ages 12 and over, becoming the first country to OK the shot for children under the age of 16. Dr. Supriya Sharma, the chief medical adviser at Health Canada, said the decision was made because the vaccine is safe and effective in that age group. It is the first coronavirus vaccine approved for children in Canada.
Fabien Paquette, Pfizer Canada vaccines lead, called the expansion in Canada "a significant step forward in helping the Canadian government broaden its vaccination program and begin to help protect adolescents before the start of the next school year," in a statement posted Wednesday.
Pfizer in March announced that a Phase 3 trial involving its coronavirus vaccine in adolescents ages 12 to 15 was found to be safe and 100% effective. The BNT162b2 vaccine produced "robust antibody responses, exceeding those recorded earlier in vaccinated participants aged 16 to 25 years old," and that it was well tolerated, company officials said at the time.
FAUCI SAYS US IS IN ‘BOTTOM OF THE SIXTH’ IN COVID-19 TIMELINE
Dr. Anthony Fauci, the country’s top infectious disease expert, in an interview Wednesday said he expected expanded authorization in the U.S. "within several days."
"I think it’s going to be very soon, I mean I don’t want to get ahead of the FDA but I believe it’s going to be within several days, I cannot imagine it’s going to be much longer than that," Fauci told NBC’s Today show co-hosts.
Further, President Joe Biden also addressed inoculation efforts among the nation’s teenagers on Tuesday while providing an update on the pandemic, should previously authorized jabs see expanded approval to include younger age groups. If the FDA grants authorization for the jabs among teenagers, the president said the administration would "immediately move" to ready 20,000 pharmacy sites nationwide in delivering shots to teens. The Biden administration also plans to ship vaccines directly to pediatricians over the following weeks.
Fox News’ Alexandria Hein and Madeline Farber and the Associated Press contributed to this report.