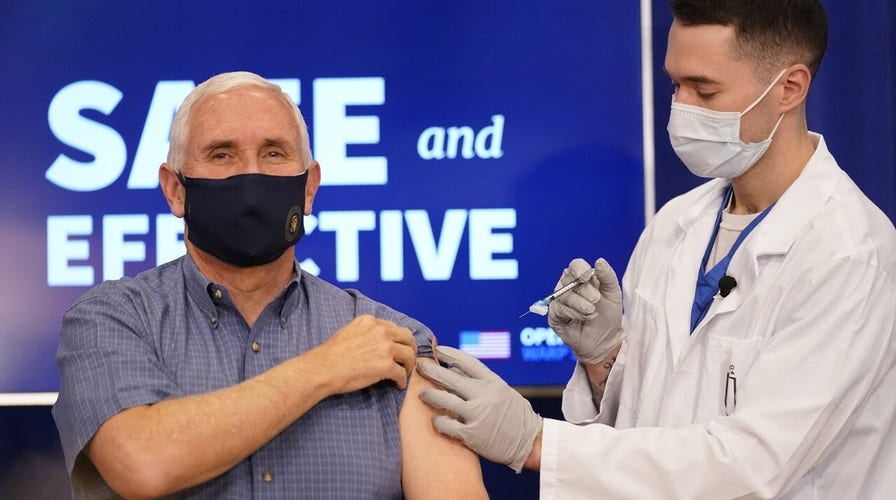
Pence receives coronavirus vaccine, praises Operation Warp Speed: 'Truly a medical miracle'
Vice President Mike Pence makes remarks as he and second lady Karen Pence receive the coronavirus vaccine.
The Food and Drug Administration issued an emergency authorization Friday for a second coronavirus vaccine produced by Moderna for people ages 18 and younger, the agency said Friday.
An independent advisory panel to the FDA voted to endorse Moderna’s shot Thursday. The U.S. surpassed 17 million total cases on Thursday, according to Johns Hopkins University.
"With the availability of two vaccines now for the prevention of COVID-19, the FDA has taken another crucial step in the fight against this global pandemic that is causing vast numbers of hospitalizations and deaths in the United States each day," said FDA Commissioner Stephen M. Hahn. "Through the FDA’s open and transparent scientific review process, two COVID-19 vaccines have been authorized in an expedited timeframe while adhering to the rigorous standards for safety, effectiveness, and manufacturing quality needed to support emergency use authorization that the American people have come to expect from the FDA."
Fast Facts
FDA advisory panel vote to endorse Moderna vaccine Thursday was 20-0. One committee member abstained.
Committee members voted to endorse the vaccine’s use in individuals ages 18 and older, while Pfizer received emergency approval last week for those ages 16 and up.
Vice President Mike Pence, his wife and U.S. Surgeon General Dr. Jerome Adams all rolled their sleeves Friday morning and received the recently approved Pfizer COVID-19 vaccine on live television.
The vaccinations were administered following a breaking report from top officials at the U.S. Food and Drug Administration that the agency is "rapidly working" to approve Moderna’s COVID-19 vaccine.
Follow below for the latest updates on the coronavirus pandemic. Mobile users click here.