With new guidelines released by the U.S. Food and Drug Administration (FDA), blood donor eligibility now will be based on individuals’ responses to screening questions — rather than group-wide restrictions.
With the previous guidelines, men who have sex with men (MSM) faced restrictions when donating blood due to concerns about the spread of HIV from donor to recipient.
With the new relaxed rules, each donor candidate will be required to answer the same set of screening questions to determine potential HIV risk "regardless of sexual orientation, sex or gender."
Eligibility then will be determined for each individual.
EVERYTHING YOU NEED TO KNOW ABOUT DONATING BLOOD DURING RED CROSS MONTH
"The FDA has worked diligently to evaluate our policies and ensure we had the scientific evidence to support individual risk assessment for donor eligibility while maintaining appropriate safeguards to protect recipients of blood products," said Dr. Peter Marks, PhD, director of the FDA’s Center for Biologics Evaluation and Research in Washington, D.C., in a press release announcing the new guidelines.
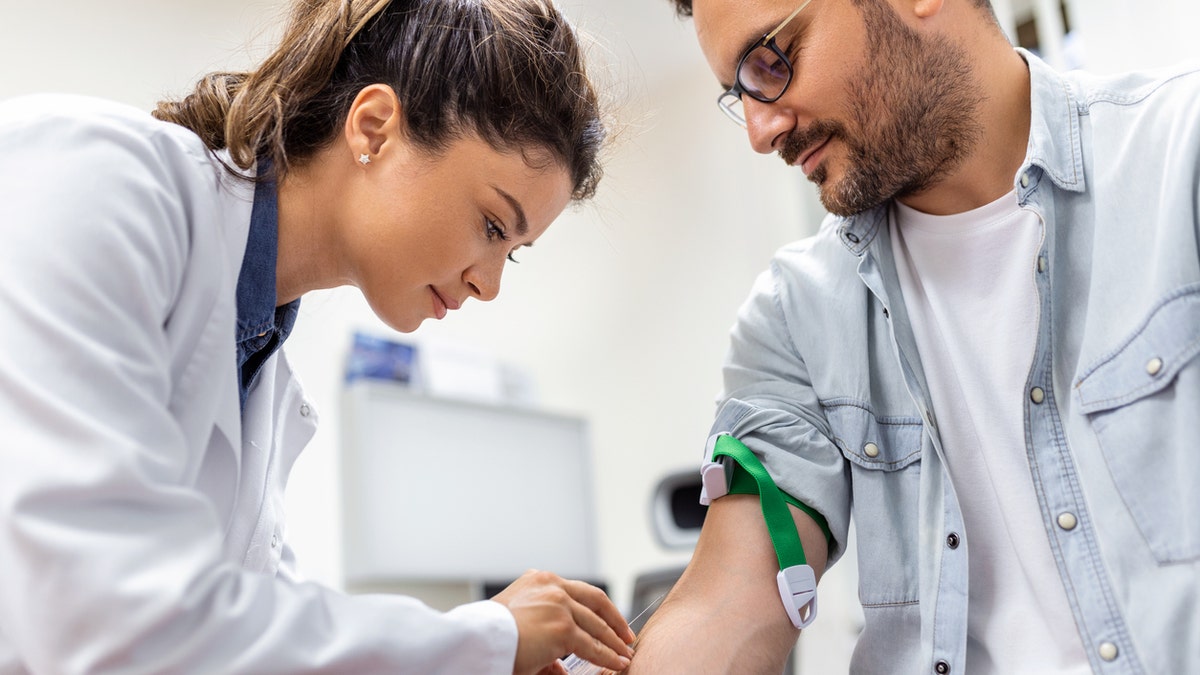
With new guidelines released by the U.S. Food and Drug Administration (FDA), blood donor eligibility will be based on individuals’ responses to screening questions — rather than on group-wide restrictions. (iStock)
"The implementation of these recommendations will represent a significant milestone for the agency and the LGBTQI+ community," he continued.
"The FDA is committed to working closely with the blood collection industry to help ensure timely implementation of the new recommendations and we will continue to monitor the safety of the blood supply once this individual risk-based approach is in place," Marks added.
How restrictions have changed for gay and bisexual men
With the start of the HIV epidemic in the U.S. in the early 1980s, men who had sex with men were not permitted to give blood for fear they would spread human immunodeficiency virus, the virus that causes AIDS (acquired immunodeficiency syndrome).
The ban continued for more than 30 years.
BLOOD DONATIONS THIS WINTER: AMERICAN RED CROSS URGES PEOPLE NOT TO FORGET TO DONATE
In 2015, in light of new testing and treatment developments, the restrictions relaxed a bit — MSM were permitted to give blood if they hadn't had sex with a man over the last 12 months.
The next change was in 2020, when the time frame for abstaining from sex with other men was reduced to three months.
Measures to ensure safe donations
Prospective blood donors will be required to fill out questionnaires to gauge their level of risk.
Anyone who reports having a new sexual partner, multiple sexual partners or anal sex over the past few months — regardless of sexual orientation — will not be permitted to donate due to the chance of recent and undetected HIV infection, per the FDA’s statement.
"The FDA is committed to working closely with the blood collection industry to help ensure timely implementation of the new recommendations and we will continue to monitor the safety of the blood supply once this individual risk-based approach is in place," said Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research. (iStock)
Anyone who is taking medications to treat or prevent HIV infection would also be "deferred" from donating.
Those medications include antiretroviral therapy (ART), pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP).
"Though these antiretroviral drugs are safe, effective and an important public health tool, the available data demonstrate that their use may delay detection of HIV by currently licensed screening tests for blood donations, which may potentially give false negative results," the FDA’s statement said.
"The issue needs to be based on medical risk, not demographics or political correctness." — Dr. Marc Siegel
"The FDA strongly believes the implementation of an individual risk-based approach will not adversely affect the safety or availability of the U.S. blood supply," the statement said.
The FDA’s new guidance is based on its review of data from countries that have implemented individual risk screenings as well as multiple studies measuring risk factors for HIV transmission during transfusions.
All donated blood is tested for certain antibodies that may cause adverse reactions in the recipient, per the Centers for Disease Control and Prevention (CDC).

Prospective blood donors will be required to fill out questionnaires to gauge their level of risk. (iStock)
It is also tested for hepatitis B and C, HIV, West Nile virus, bacterial contamination and any other pathogens.
"I applaud the FDA's decision to follow science and not the biases that have guided previous blood donation policy with their new recommendations that apply to all Americans, regardless of sexual orientation," Dr. David Kilmnick, president of the New York LGBT Network, told Fox News Digital.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
"Donating blood is a selfless act that helps to save lives, and this new ruling is a major step toward ending the discriminatory policy on blood donations that was rooted in fear and homophobia," he also said.
"With our blood supply at a critical shortage, this ruling could not wait a day longer and will help to save lives."
CLICK HERE TO GET THE FOX NEWS APP
Dr. Marc Siegel, a professor of medicine at NYU Langone Medical Center and a Fox News medical contributor, advised caution, however.
"The issue needs to be based on medical risk, not demographics or political correctness," he told Fox News Digital. "Because you could have a virus and test negative initially and then turn positive later, so it's not foolproof."
He added, "My suggestion would be to consider allowing it, but only with serial testing for these common diseases."




















