Doctors raise alarms over shortage of cancer drugs: 'This is an emergency'
Fox News medical contributor Dr. Marc Siegel argues the answer to the shortage is government intervention and calls on the FDA to work with manufacturers to increase supplies.
While a breast cancer vaccine is not yet approved for widespread use, there are trials underway — including one at Cleveland Clinic, where 46-year-old Jennifer Davis of Ohio was the first person to get the shot in 2021.
The vaccine had been in development at Cleveland Clinic for more than 20 years before it finally reached the human trial phase. Now, researchers are hopeful it could be available to certain cancer survivors within a few years.
In an interview with Fox News Digital, Davis said the vaccine has brought her peace of mind that the disease could be behind her for good.
OHIO WOMAN PUSHES PAST BREAST CANCER, WON'T LET DIAGNOSIS SLOW HER DOWN
"It all fell into place and worked out perfectly," she said — though her journey is not over yet.
A long time coming
The breast cancer vaccine has been licensed to Anixa Biosciences, which is working with Cleveland Clinic on the rollout. Fox News Digital spoke with Dr. Amit Kumar, CEO of Anixa, about the extended journey to bring the vaccine to trial.
Kumar explained that Dr. Vince Tuohy, an immunologist at the Cleveland Clinic, invented the vaccine that's currently being tested.

Jennifer Davis of Ohio is pictured here with photos of herself during her cancer journey. She was the first person to receive a breast cancer vaccine in 2021 at Cleveland Clinic. Now, she's awaiting the results. (Jennifer Davis)
"Vince ran the research group that conducted the research on this vaccine for two decades," Kumar said. "He was a great scientist and we became good friends as we worked together. Unfortunately, he passed away a few weeks ago at the age of 74."
Now, Tuohy’s fellow researchers — including Dr. G. Thomas Budd and Dr. Justin Johnson — are continuing work on the vaccine, in collaboration with Kumar and his team.
Ohio cancer survivor was first to be vaccinated
Davis is a nurse who has three adult children. She was initially diagnosed with triple-negative breast cancer in 2018.
Triple-negative is a more aggressive type of breast cancer that does not have any of the three common "receptors" in the cells, which means it doesn’t respond to the hormonal therapies that are typically used to fight the disease.
About 15% of all breast cancers fall into this category.
Triple-negative breast cancer is more aggressive and harder to treat.
Davis had a rigorous round of treatments that included chemotherapy, surgery and 26 rounds of radiation. While the treatment worked and she was pronounced cancer-free, she was still concerned.
"Triple-negative cancer is so aggressive and recurrence is really, really high — the prognosis is not the greatest," she told Fox News Digital.
"And there was nothing I could take following treatment. Once treatment is over, there's no pill or anything that gives you that peace of mind that it's not going to come back."

Davis, pictured with a photo of herself receiving the vaccine, said the shot has given her peace of mind and reduced her worries about her breast cancer coming back. (Jennifer Davis)
When Davis heard about the vaccine trial at Cleveland Clinic, she applied and was thrilled to get picked.
"There were very specific guidelines and a lot of testing I had to go through," she said.
"I was so close to not being able to get it," she also said, adding that "it all fell into place and worked out perfectly."
NEW BREAST CANCER GENE CAN PREDICT LIKELIHOOD OF HEREDITARY DISEASE, STUDY FINDS
Davis received her first dose of the vaccine on Oct. 19, 2021. After that, she received two additional doses spaced two weeks apart.
Then began the long wait to find out whether the shots worked.
Hoping for good news
The vaccine has been administered to 14 patients so far, Dr. Kumar said.
Next week, at the annual meeting of the American Association of Cancer Research, the research team will present the scientific data for the first group of patients.
"The data is looking very good so far," the doctor said.

Before getting the vaccine, Davis (pictured with her husband) went through grueling treatments that included chemotherapy, surgeries and 26 rounds of radiation. Of the breast cancer vaccine she received during the trial period, she said, "There were very specific guidelines and a lot of testing I had to go through." Now, she's awaiting the results. (Jennifer Davis)
As Davis explained, she had lab work done before and after each vaccine dose.
She has not yet seen the results of those tests — so she's looking forward to the presentation next week.
"I’m very, very excited," she said. "What I'm waiting to find out, what everybody is looking to see, is whether I built up an immune response to the breast cancer."
"Every woman in the world could potentially be a candidate for this vaccine."
If the results are as positive as she hopes, Davis looks forward to a time when more women will have access to the vaccine — not just those who have already been treated for triple-negative cancer, like her, but also healthy women who want to prevent cancer from developing in the first place.
"On the grander scale, this could eliminate triple-negative breast cancer," she said.
"If that piece of the puzzle could be completely removed and we never had to worry about it again, that would be amazing."
Looking ahead with optimism
Today, Davis is just six months away from being cancer-free for five years, a milestone she doesn’t take lightly.
She said receiving the vaccine has given her a sense of hope that might not otherwise have been as easy to come by.
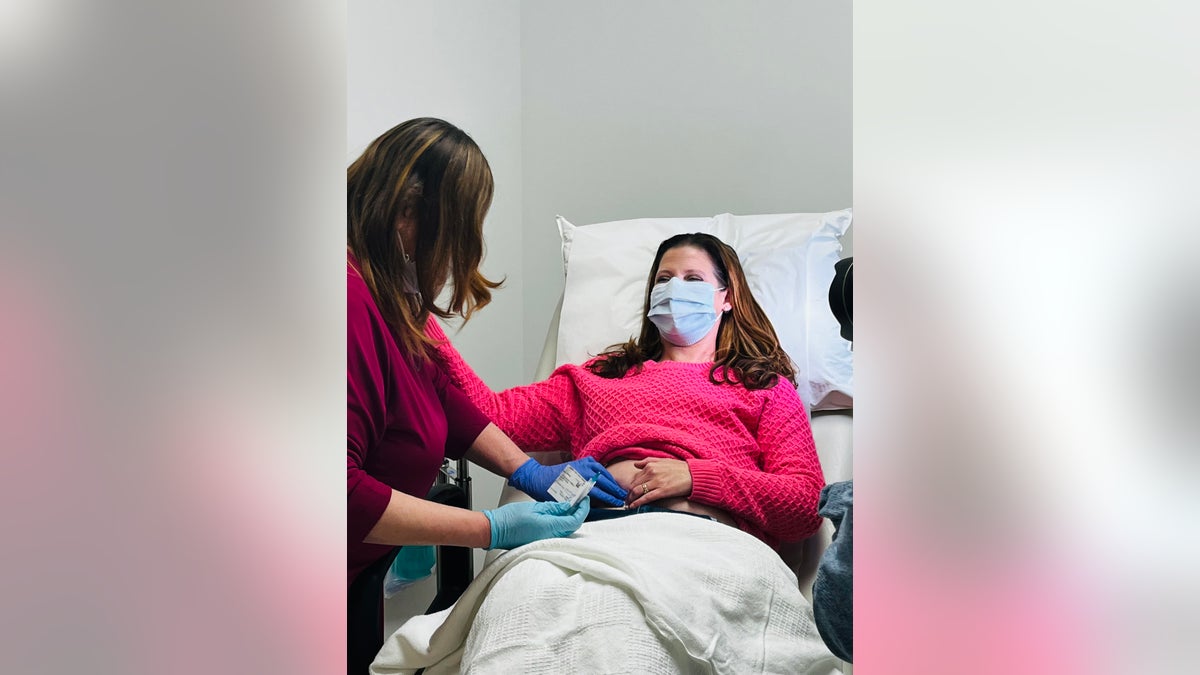
Davis (shown here with mask on) looks forward to a time when more women will have access to the vaccine — not just those who have already been treated for triple-negative breast cancer, but also healthy women who want to prevent cancer from developing in the first place. (Cleveland Clinic)
"It used to be that for every little headache, I would think I might have a brain tumor, and if my arm started to hurt, I’d think I had bone cancer," she said. "There was that constant worry that something has turned into something else. But after the vaccine, even though I don't know the results of it yet, I don’t have that worry anymore, which has been wonderful."
Kumar said he is also hopeful the vaccine will eliminate that anxiety in all women.
"I’d love to be able to give this vaccine to my daughters to reduce their risk."
"In the U.S., there are 3.6 million women who are breast cancer survivors, and they wake up every morning worried that their cancer is going to come back," he said.
"We would love to be able to give all of those women a shot so they don’t have to worry about cancer recurrence."
FDA ISSUES NEW MAMMOGRAM REGULATIONS AIMED AT FURTHER BREAST CANCER PREVENTION
This is especially important for triple-negative cancer, which is usually much more aggressive if it comes back, he said.
The doctor foresees the vaccine's eventual availability to all women, even those without prior breast cancer diagnoses.
The breast cancer vaccine was in development at Cleveland Clinic for more than 20 years before finally reaching the human trial phase. (iStock)
"Once we've completed all the appropriate clinical studies, every woman in the world could potentially be a candidate for this vaccine," he said.
"We believe the vaccine will be available within four to five years for recurrence prevention and then for primary prevention a few years after that," the doctor added.
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
"Recurrence prevention" focuses on people like Davis, who have previously had breast cancer and face a high risk of recurrence, Kumar explained.
"Primary prevention" refers to the vaccination of women who have never had cancer to help ensure they don't get it.
CLICK HERE TO GET THE FOX NEWS APP
"I have two daughters, and our family has a history of breast cancer," Kumar said. "I’d love to be able to give this vaccine to my daughters to reduce their risk."
Breast cancer is the world’s most common type of cancer, with 2.3 million women diagnosed in 2020, per the World Health Organization.