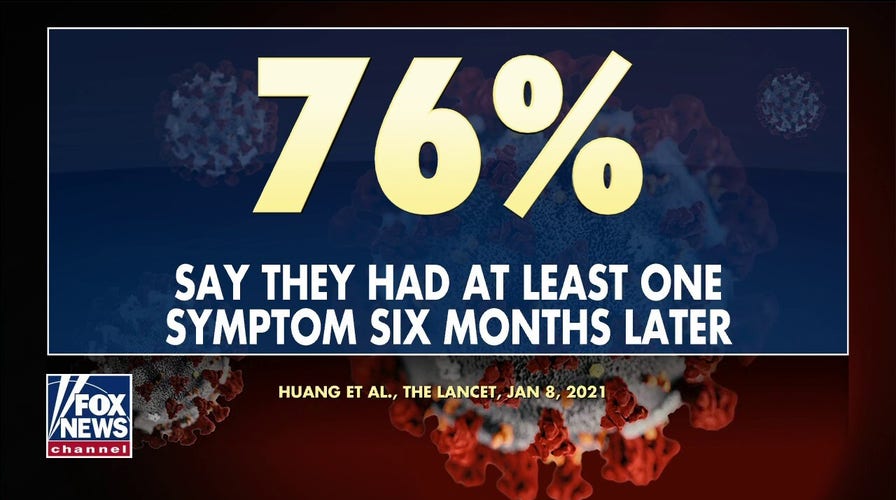
Study out of Wuhan, China, claims coronavirus symptoms can last at least 6 months
Dr. Marc Siegel discusses potential long-term symptoms of the virus and President-elect Biden’s vaccine rollout plan.
As experts warn about the high transmissibility of a strain of coronavirus first detected in the U.K. several weeks ago, many have voiced concern about whether currently approved vaccines and treatments will remain effective. Remdesivir, which became the first FDA-approved treatment for COVID-19 last fall, should be effective against new strains, Gilead Sciences CEO told CNBC on Monday.
The effectiveness of the antiviral drug on the current strain has been debated as some studies found that it has no impact on death rates from disease, while others suggest that it could be highly effective in some patients. The FDA approved it for use in adult and pediatric patients 12 years and older after clinical trials showed that it shortened hospital stay and recovery time in patients with mild-to-severe COVID-19.
The U.K. variant, identified as B.1.1.7, has showed to be more transmissible, but it is not believed to cause more severe illness than the previously identified coronavirus. Pfizer and BioNTech has vouched for the effectiveness of their vaccine against the variant, and have touted the "flexibility" of mRNA technology should a tweak be needed.
5 CASES OF UK CORONAVIRUS VARIANT FOUND IN MINNESOTA
But previously, experts have said that therapeutics are far more complicated to develop than vaccines. Research is ongoing, but for now, Gilead Sciences CEO Daniel O’Day has voiced confidence in remdesivir’s ability to remain effective against the U.K. coronavirus strain because of the way it works.
PFIZER COVID-19 VACCINE BELIEVED TO BE EFFECTIVE AGAINST NEW VARIANT
"Remdesivir works at the source in the cell where the virus replicates, and what we know is in these new variants, that part of the cell is not changing at all in fact," O’Day told "Squawk Box." "So, we fully expect remdesivir to be effective against these new strains."
CLICK HERE FOR COMPLETE CORONAVIRUS COVERAGE
O’Day told the news outlet that the company has already tested the drug against 2,000 samples of strains in laboratories, and each time it "maintained its effectiveness."