New Jersey biotech company uses artificial intelligence for drug development
PsychoGenics CEO Emer Leahy of Paramus, New Jersey, explains how the first potential AI-discovered treatment for schizophrenia was developed through machine learning. Fox News Digital spoke with her.
As the world of artificial intelligence continues to evolve, a New Jersey biotech company is taking AI capabilities to the next level.
After decades of working with AI-driven phenotypic platforms in an attempt to develop drugs for mental illness, PsychoGenics has had a breakthrough with one compound that aims to treat schizophrenia.
PsychoGenics president and CEO Emer Leahy spoke to Fox News Digital in a recent on-camera interview, explaining that she and her team in partnership with Sunovion are closer than ever to seeing what she believes is the first-ever AI-discovered drug.
AI-POWERED MENTAL HEALTH DIAGNOSTIC TOOL COULD BE THE FIRST OF ITS KIND TO PREDICT, TREAT DEPRESSION
"This is the first de novo discovered [drug], I believe, to hit the market," she said.
Scientists at Yale University’s Department of Psychiatry founded PsychoGenics in 1999. They first worked with "transgenic mouse models" to better understand and respond to psychiatric disorders, Paramus-based Leahy explained.

Emer Leahy is president and CEO of PsychoGenics, a New Jersey biotech company. They employ AI to discover treatments for mental illness. (PsychoGenics)
"We essentially got started with the mission of looking at the behavior of these genetically altered mice for neuropsychiatric disorders," she said.
"Then we started providing a service to pharma and biotech generally."
AI TOOL GIVES DOCTORS PERSONALIZED ALZHEIMER'S TREATMENT PLANS FOR DEMENTIA PATIENTS
PsychoGenics has since expanded its capabilities to offer a "broad suite" of pre-clinical offerings, Leahy said, to support central nervous system (CNS) drug discovery.
In 2002, when PsychoGenics was only a team of eight, the company took an advanced dive into discovering new drugs based on its expertise in the behavioral testing of rodents.
Leahy has worked in pharma and biotech for more than 30 years. She said she pushed her team to "industrialize" its method.
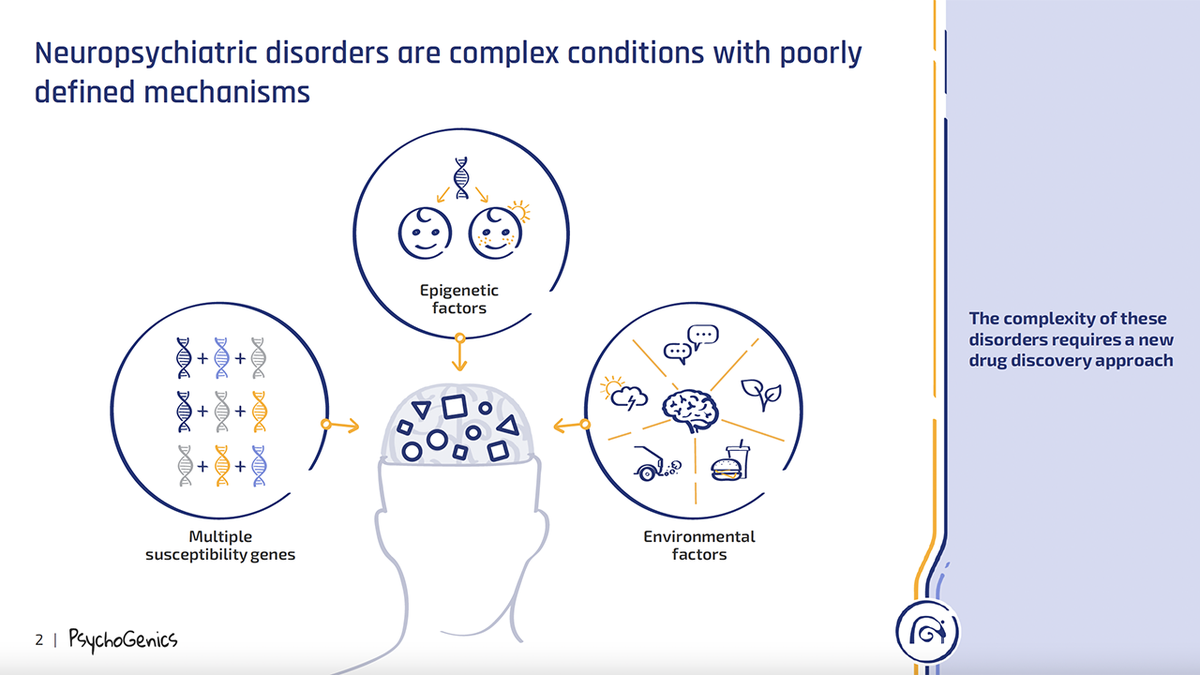
This illustration from PsychoGenics of New Jersey shows the complexity of neuropsychiatric disorders. (PsychoGenics)
"It was the peak of the genomic revolution," she said. "Everybody was chasing targets and genes — and essentially what I was saying was, 'Let’s not do that. Let’s use behavior, the output of the brain, to drive the discovery efforts, and let's look for patterns in behavior that will allow us to predict new treatments.'"
The then-small PsychoGenics team took its "big ideas" to Carnegie Mellon University in Pittsburgh, Pennsylvania, in search of high-tech tools to develop the first platform, which is now known as SmartCube.
BRAIN HEALTH EXPERT ADDRESSES LONELINESS EPIDEMIC: HERE ARE SIMPLE WAYS TO FIGHT IT
She said that SmartCube "has been responsible for bringing a number of treatments to clinical trials."
The most advanced of those to come out of SmartCube is Ulotaront, which sets out to treat schizophrenia.
The drug is being developed by Sunovion Pharmaceuticals and is in phase three trials, which is when it's monitored for "efficacy and monitoring of adverse reactions," according to the FDA.
How the AI-powered SmartCube works
SmartCube combines robotics, computer vision and artificial intelligence in a box-shaped mechanism.
The cube presents its subjects (drug-injected mice) with a series of challenges to influence their behavior.
"The floor will change configuration, [the mouse] will get an air puff which will startle him," Leahy explained. "Different things happen over the course of an hour."
She detailed how multiple cameras within the box capture "everything that the mouse is doing in session."

Millions of data points are condensed down into a couple of thousand "behavioral features," from which, Leahy explained, a drug signature is extracted. (iStock)
"We’re collecting a couple million data points in a session," she said. "You can’t employ parametric statistics to make sense of that sort of data, so we use machine learning."
Those millions of data points are then condensed down into a couple of thousand "behavioral features," from which, Leahy explained, a drug signature is extracted.
"We can identify the behavioral features that are prominent in the animal in response to a different treatment," she said.
CEO Leahy mentioned that PsychoGenics has built a "reference database."
It is based on a variety of other compounds — such as antidepressants, anxiolytics, mood stabilizers and other anti-psychotics — to compare to the new drug being tested.
"We can test thousands of compounds in SmartCube, and we can predict their potential therapeutic utility," she said.

"There’s a real need for a better treatment for schizophrenia," said Leahy, the CEO of PsychoGenics. The company is working to fill a need in the untapped market of schizophrenia treatments, it shared with Fox News Digital. (iStock)
Leahy revealed that about 40% of compounds will show a "signature" that indicates its potential for a new drug discovery program.
Ulotaront is a "great example" of how this process works, Leahy added, as PsychoGenics sought to fill a need in the untapped market of schizophrenia treatments.
"There’s a real need for a better treatment for schizophrenia," she said.
AI HEALTH CARE PLATFORM PREDICTS DIABETES WITH HIGH ACCURACY BUT ‘WON’T REPLACE PATIENT CARE'
All antipsychotics that are currently available work by targeting D2, the dopamine receptor for antipsychotic drugs, according to Leahy — and there have been no new mechanisms in about 60 years.
PsychoGenics and Sunovion were able to go a "step further" by the discovery of a schizophrenia drug that did not target D2.

A patient has a therapy session with a psychologist. "Existing treatments," said Leahy, "have minimal impact on what’s called the ‘negative symptoms’ of schizophrenia … the apathy, the social withdrawal, the flat affects. They’re addressing ‘positive symptoms’ — the hearing of voices, the psychosis — but not the ‘negative symptoms,’ which are very disabling." (iStock)
The problem with D2 is that about one-third of patients do not respond to it, Leahy explained.
"These existing treatments have minimal impact on what’s called the ‘negative symptoms’ of schizophrenia … the apathy, the social withdrawal, the flat affects," she said.
"They’re addressing ‘positive symptoms’ — the hearing of voices, the psychosis — but not the ‘negative symptoms,’ which are very disabling."
TEENS ARE TURNING TO SNAPCHAT'S 'MY AI' FOR MENTAL HEALTH SUPPORT — WHICH DOCTORS WARN AGAINST
Patients also sometimes refuse these treatments due to side effects, which can often lead to relapse, said Leahy.
As the chemists worked to identify several potential drug "hits," in partnership with Sunovion, PsychoGenics ended up with "just a handful of compounds" that fit the bill, Leahy said.
"We ultimately identified Ulotaront," Leahy said.
"And we predicted that Ulotaront would have effects on negative symptoms, and it would have a better safety profile."

If the results of phase three trials are positive, Ulotaront could come to market in 2024. "We’re pretty excited about this," Leahy said. "It’s going to deliver what we believe is a better treatment for patients who suffer from this severely disabling condition." (iStock)
Ulotaront had an "outstanding result" after phase two clinical trials, according to Leahy, showing it could improve both positive and negative schizophrenia symptoms.
PsychoGenics expects the compound to "read out" on multiple phase three trials later this year, which means the data will be made public.
AI LIFE HACKS: HOW TRAVELERS ARE USING CHATGPT TO PLAN TRIPS ON A BUDGET
If the results are positive, Ulotaront could come to market in 2024.
"We’re pretty excited about this," Leahy said. "It’s going to deliver what we believe is a better treatment for patients who suffer from this severely disabling condition."
Artificial intelligence in health
Leahy said the industry has seen an "explosion" of AI usage in drug development just over the last few years.
AI is "only as good as the data you give it."
With the advent of SmartCube, PsychoGenics became one of the first health care companies, if not the first, to employ AI in the drug discovery space, according to Leahy.
"Instead of what is typically 2,500 compounds tested, before you get to nominate a compound to go into toxicology studies and clinical trials, we’re getting there in about 300," she said.
CCLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
"We’re getting there in about less than three years compared to about five-plus years, and at a fraction of the cost of other approaches."
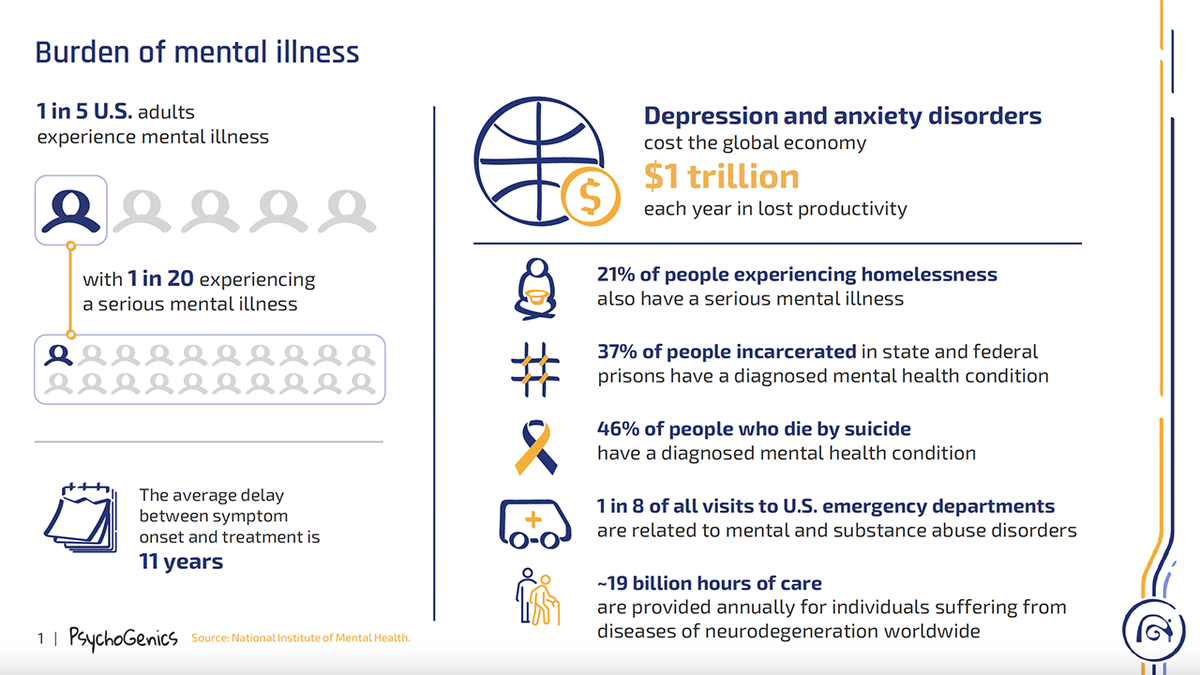
This informational guide provided by PsychoGenics details the cost of mental health in America, according to the National Institute of Mental Health. (PsychoGenics)
After decades of dealing with advanced technology and machine learning, Leahy noted that AI is "only as good as the data you give it."
She added, "What we’re doing … is looking at patterns — patterns that a human being cannot do. A human being cannot take 2,000 behavioral features and distill that into a drug signature and then make predictions … So it’s a very powerful tool. We’re going to see tremendous growth."
CLICK HERE TO GET THE FOX NEWS APP
Leahy said there’s "enormous potential" for improvement within the mental health space, as AI has the capabilities to mine literature to develop new hypotheses and identify potential new treatments.









































