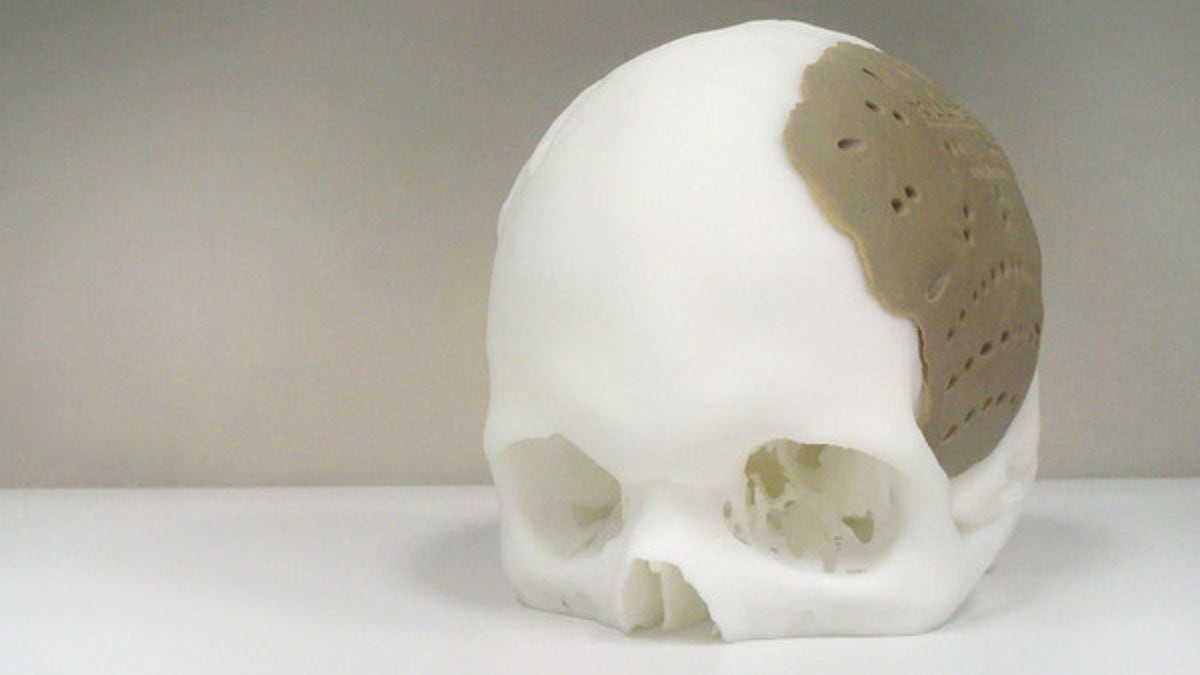
(OsteoFab)
3D printing technology has helped replace 75 percent of a patient's skull with the approval of U.S. regulators.
The 3D-printed implant can replace the bone in people's skulls damaged by disease or trauma, according to Oxford Performance Materials. The company announced it had received approval from the U.S. Food and Drug Administration for its skull implant on Feb. 18 — a decision that led to the first U.S. surgical operation on March 4
"We see no part of the orthopedic industry being untouched by this," said Scott DeFelice, president of Oxford Performance Materials.
[pullquote]
DeFelice's company is already selling 3D-printed implants overseas as a contract manufacturer. But the FDA decision has opened the door for U.S. operations using the implants. [Video: A 3D Printer of Your Own]
3D printing's advantage comes from taking the digitally scanned model of a patient's skull and "printing" out a matching 3D object layer by layer. The precise manufacturing technique can even make tiny surface or edge details on the replacement part that encourage the growth of cells and allow bone to attach more easily.
About 300 to 500 U.S. patients could use skull bone replacements every month, according to DeFelice. The possible patients include people with cancerous bone in their skulls, as well as car accident victims and U.S. military members suffering from head trauma.
DeFelice envisions going beyond the OsteoFab Patient Specific Cranial Device to make 3D-printed bone replacements for all parts of the human body. His company has already begun preparing to submit other 3D-printed bone parts for FDA approval — a huge market worth as much as $50 million to $100 million for each bone replacement type.
"If you can replace a bony void in someone's head next to the brain, you have a pretty good platform for filling bony voids elsewhere," DeFelice told TechNewsDaily.
Oxford Performance Materials adapted EOS P800 printing technology to use a special polyetherketoneketone (PEKK) material that has proved suitable for human implants. The company runs a biomedical-compliant manufacturing facility in South Windsor, Conn., that can print bone replacements fitted for specific patients in two weeks or less.
Such possibilities represent just one small part of 3D printing's potential to revolutionize U.S. manufacturing and innovation. Oxford Performance Materials is one of many companies and universities that helped found the U.S. National Additive Manufacturing Innovation Institute — a $30 million pilot institute funded by the U.S. government to help transform 3D printing into a serious manufacturing tool.
