Elon Musk, media warn of dangers surrounding AI
FOX Business senior correspondent Charlie Gasparino joined 'MediaBuzz' to discuss growing concerns surrounding the future of artificial intelligence.
In a remarkable leap forward for neuroscience and technological innovation, Elon Musk's brain implant company Neuralink has officially received approval from the FDA to begin the first human trials of its groundbreaking brain-computer interface.
The announcement was made via Twitter. However, no details were given at the time of publishing about when the clinical trials or recruitment would begin.
What does this new invention do?
Neuralink's overall goal is to have the human nervous system be able to communicate with computers. The invention it is developing is called the Neuralink N1 implant. It is slightly larger than a quarter.

Neuralink's goal is for the human nervous system to be able to communicate with computers. (Neuralink)
Here is where it gets a little creepy. The device is designed to replace a small chunk of a human skull and fit completely underneath a person's skin. Once the device is in place, its 64 needle probes are inserted into the brain, which then allows 1,024 channels of two-way communication between the brain and a computer chip.
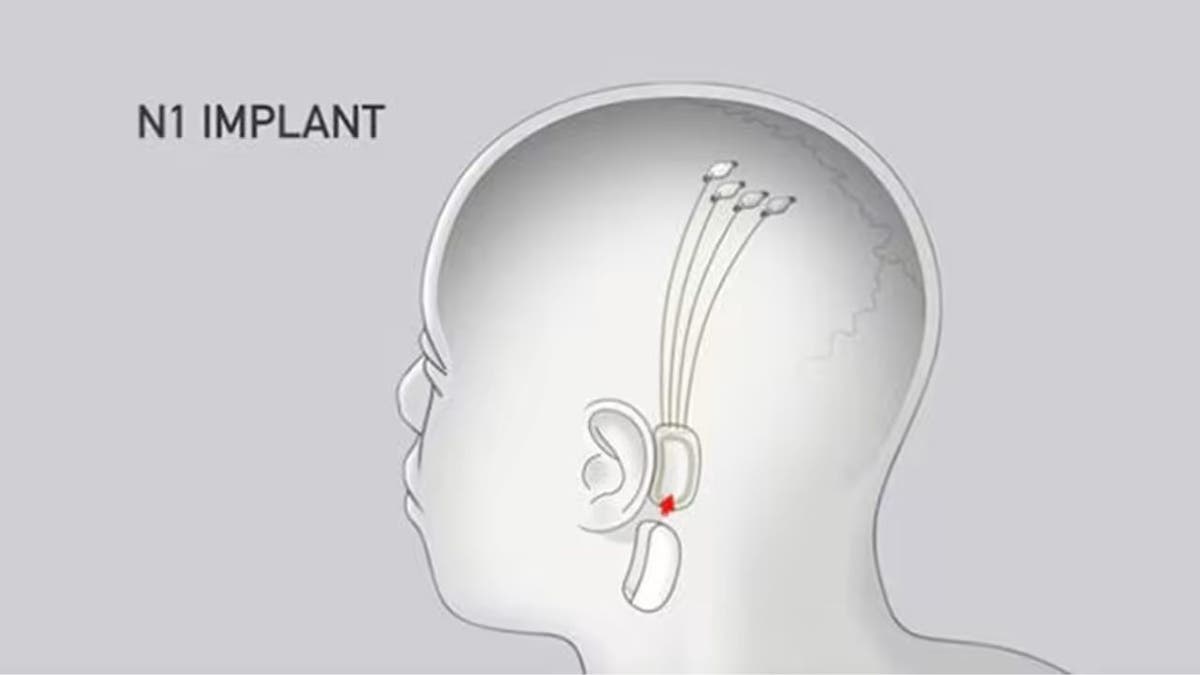
Here is a drawing showing how the device would be implanted in a person's head. (NeuraLink)
Once that communication channel is set, then the device can communicate from the human brain to external devices, which would basically allow a person to communicate with a device like an iPhone, for example, using just their mind. The device can be charged wirelessly as well.
OPENAI AND FIGURE DEVELOP TERRIFYINGLY CREEPY HUMANOID ROBOTS FOR THE WORKFORCE
What will the N1 implant be used for?
The plan is to start using the device only with quadriplegic patients first. Musk said he's wanted to see if the device will help people restore their vision as well as people with little or no ability to operate their muscles rapidly to operate computers and control devices. He also said he hoped the device could be used to reconnect the brain's communication with the spinal cord for someone with a broken neck.
Once clinical trials take place, and there is a better idea of how safe and secure this device is, Musk aims to put it on the market for the public. He said his brain-computer interfaces could have the potential to restore independence and improve lives for the better.
Is this device safe to use?
Well, it has yet to be determined if the device is safe because its clinical trials have not yet begun. The possibilities for what this device can do, however, are endless. This could potentially be used in the future to control bionic limbs, think of a message and send it directly to someone else's neural implant, play video and audio without the need for displays and more.
The main concern that most have is how exactly the device will be inserted safely. Neuralink has created a robot called R1 that was designed specifically to implant these chips safely without removing the brain's protective outer layer.
Has this ever been attempted before?
What is interesting is that another company, Blackrock Neurotech, has already inserted its own brain implant into patients over 50 times since 2004 with much success. Their chip, known as the NeuroPort Array, does poke out through the skin, so it isn't hidden as well as the N1 implant plans to be.
However, it has worked extremely well with quadriplegic patients helping them regain tactile function, movement of limbs and prosthetics and the ability to control digital devices. It gives hope that Musk's N1 implant might really work. Below is a video from Blackrock Neurotech showing how their chip has been successful.
Can I be part of the Neuralink clinical trials?
If you are interested in learning whether you may qualify for future Neuralink clinical trials, you can join their patient registry by logging on here. You will have to fill out a brief screener to determine whether you are eligible to submit a patient registry application. Their website states that:
"Anyone within the United States who is at least 18 years old and the age of majority in their state, who is able to consent, and who has quadriplegia, paraplegia, vision loss, hearing loss, and/or the inability to speak, is invited to participate in the Patient Registry."
ROBOTS COULD GO FULL 'TERMINATOR' AFTER SCIENTISTS CREATE REALISTIC, SELF-HEALING SKIN
Kurt's key takeaways
This could be a potentially life-altering invention for the world. I'm anxious to see how the clinical trials go. On the one hand, if managed responsibly and ethically, the accessibility of Neuralink-like devices could revolutionize how we interact with technology and improve the lives of individuals with various neurological conditions. However, on the other hand, concerns about the potential for misuse cannot be ignored. I believe it is crucial to approach its accessibility and deployment with extreme caution.
CLICK HERE TO GET THE FOX NEWS APP
Under what circumstances would you be willing to have Neuralink implanted in you? Let us know by writing us at CyberGuy.com/Contact.
For more of my security tips, subscribe to my free CyberGuy Report Newsletter by heading to CyberGuy.com/Newsletter.
Copyright 2023 CyberGuy.com. All rights reserved.