(Consumer Product Safety Commission)
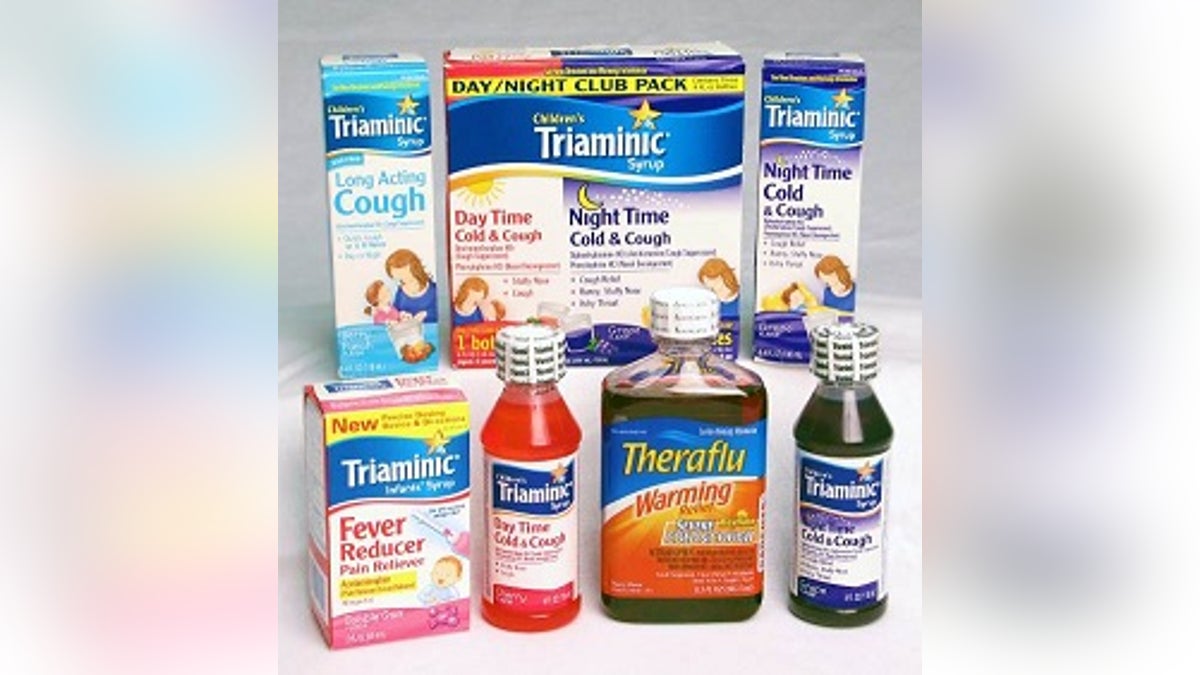
Novartis AG said on Thursday it is recalling 183 lots of cough syrup after discovering the child-resistant feature on some bottle caps was not functioning correctly.
The Swiss drug company is recalling 142 lots of Triaminic and 41 lots of Theraflu Warming Relief Syrups manufactured in the United States before December 2011.
The company said it received four reports of accidental ingestion of the Triaminic syrup. One patient required medical attention but recovered.
No adverse affects were reported with the Theraflu syrup, but the product is being recalled because it has the same cap as the malfunctioning Triaminic bottles.
The affected cough syrup bottles were produced at Novartis' Lincoln, Nebraska, manufacturing facility. A consumer complaint last November triggered an internal investigation that led the company to issue the recall.
Julie Masow, a spokeswoman for Novartis, said 97 percent of the product in question has either been used or already returned.
Manufacturing at the Lincoln, Nebraska, facility was suspended in December 2011 and has yet to reopen, Masow said.







































